Готовые презентации на тему:
- Образование
- Искусство и Фото
- Наши презентации
- Авто/мото
- Технологии
- Бизнес и предпринимательство
- Карьера
- Данные и аналитика
- Дизайн
- Устройства и комплектующие
- Экономика и Финансы
- Машиностроение
- Развлечения и Юмор
- Путешествия
- Eда
- Политика
- Юриспруденция
- Здоровье и Медицина
- Интернет
- Инвестиции
- Закон
- Стиль жизни
- Маркетинг
- Мобильные технологии
- Новости
- Недвижимость
- Рекрутинг
- Розничная торговля
- Таможня, ВЭД, Логистика
- Наука
- Услуги
- Программное обеспечение
- Спорт
- Музыка
- Шаблоны презентации
- Детские презентации
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- История
- Литература
- Информатика
- Математика
- Обществознание
- Русский язык
- Физика
- Философия
- Химия
- МХК
- ОБЖ
- Окружающий мир
- Педагогика
- Технология
- Начальная школа
- Раскраски для детей
- Товароведение
- Менеджмент
- Страхование






![Occurrence
The Sun has a concentration of 0.1 parts per billion of beryllium. Beryllium has a concentration of 2 to 6 parts per million in the Earth's crust. Beryllium is found in over 100 minerals,but most are uncommon to rare. The more common beryllium containing minerals include:
bertrandite (Be4Si2O7(OH)2)
beryl (Al2 [Be3(Si6O18)]
chrysoberyl (Al2BeO4)
phenakite (Be2SiO4).](/documents_6/f9d7299ef6e368f197b4a96e9c58f944/img5.jpg)





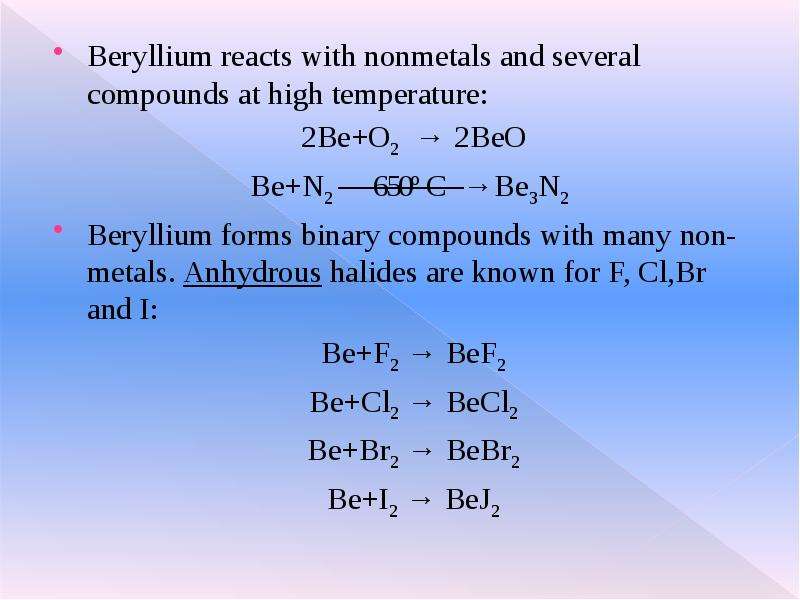
![Since beryllium is an amphoteric metal it also reacts with strong bases and liberates H2 gas
Since beryllium is an amphoteric metal it also reacts with strong bases and liberates H2 gas
Be+NaOH → Na2BeO2+H2 ↑
Be +2NaOH+2H2O → Na2 [Be(OH) 4] +H2 ↑](/documents_6/f9d7299ef6e368f197b4a96e9c58f944/img12.jpg)
![Compounds
Beryllium oxide
Beryllium oxide, BeO, is a white refractory solid, which has the wurtzite crystal structure and a thermal conductivity as high as in some metals. BeO is amphoteric.
BeO+ 2HCl (conc) → BeCl2+H2O
BeO+ 2NaOH (conc) +H2O →Na2[Be(OH) 4]](/documents_6/f9d7299ef6e368f197b4a96e9c58f944/img13.jpg)



![Beryllium sulphide reacts with hot solutions of alkali and alkali metal carbonates:
Beryllium sulphide reacts with hot solutions of alkali and alkali metal carbonates:
BeS+4NaOH →Na2 [Be(OH) 4]+Na2S
BeS +2Na2CO3+H2O →Na2 [Be(OH)6 ]+ Na2S+CO2
Halogens, with the exception of iodine (which does not react with beryllium sulphide) form halides in the interaction with BeS:
BeS+Cl2 → BeCl2+S](/documents_6/f9d7299ef6e368f197b4a96e9c58f944/img17.jpg)