Готовые презентации на тему:
- Образование
- Искусство и Фото
- Наши презентации
- Авто/мото
- Технологии
- Бизнес и предпринимательство
- Карьера
- Данные и аналитика
- Дизайн
- Устройства и комплектующие
- Экономика и Финансы
- Машиностроение
- Развлечения и Юмор
- Путешествия
- Eда
- Политика
- Юриспруденция
- Здоровье и Медицина
- Интернет
- Инвестиции
- Закон
- Стиль жизни
- Маркетинг
- Мобильные технологии
- Новости
- Недвижимость
- Рекрутинг
- Розничная торговля
- Таможня, ВЭД, Логистика
- Наука
- Услуги
- Программное обеспечение
- Спорт
- Музыка
- Шаблоны презентации
- Детские презентации
- Английский язык
- Астрономия
- Алгебра
- Биология
- География
- Геометрия
- История
- Литература
- Информатика
- Математика
- Обществознание
- Русский язык
- Физика
- Философия
- Химия
- МХК
- ОБЖ
- Окружающий мир
- Педагогика
- Технология
- Начальная школа
- Раскраски для детей
- Товароведение
- Менеджмент
- Страхование








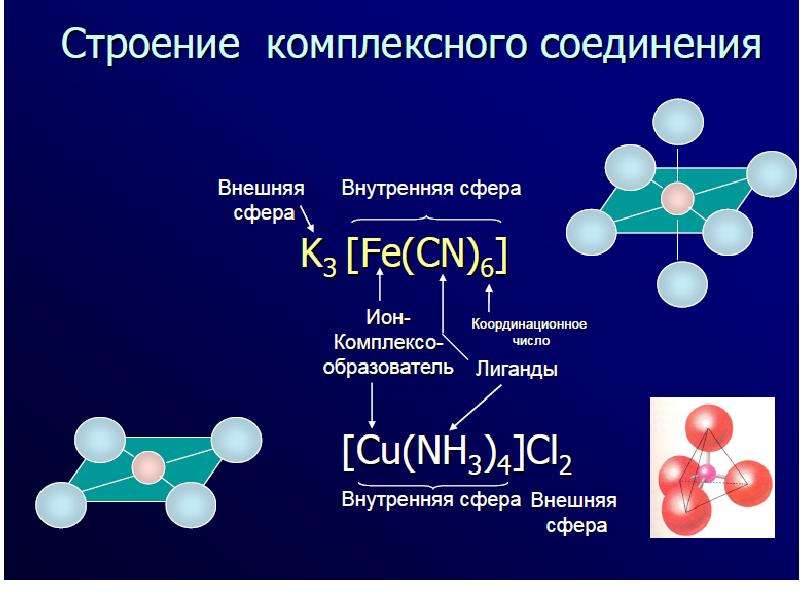


![[Cu(NH3)4]Cl2 – tetraammine copper(II) chloride;
[Cu(NH3)4]Cl2 – tetraammine copper(II) chloride;
K2 [Cu(OH)4] – potassium tetrahydroxocupprate(II);
[Cr(NH3)3Cl3] – trichloro triammine chromium(III).](/documents_6/ec394fe2962c5c2a163839d96200d6bc/img11.jpg)

![Classification of complex compounds
1. Depending upon a charge of the inner sphere:
(i) Cationic complexes (the inner sphere is positively charged – complex cations). Examples: [Cr(H2O)6]Cl3, [Co(NH3)6]Cl3.
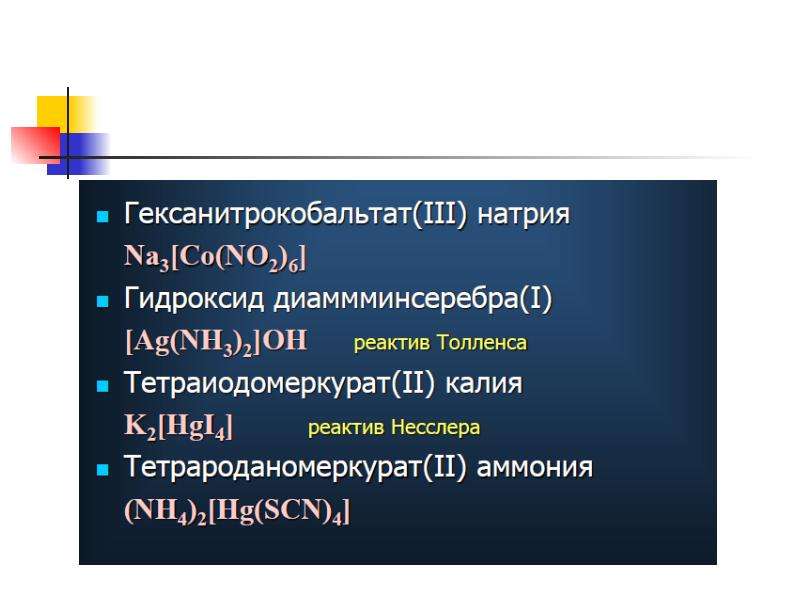
(ii) Anionic complexes (the inner sphere is negatively charged – complex anions). Examples: K2[HgI4], Na[Sb(OH)6].
(iii) Neutral complexes (the inner sphere is not charged). Examples: [Pt(NH3)2Cl2], [Fe(CO)5].](/documents_6/ec394fe2962c5c2a163839d96200d6bc/img14.jpg)
![22) Depending upon the type of the ligand:
22) Depending upon the type of the ligand:
(i) Aqua-complexes (ligands are water molecules – [Cu(H2O)5]SO4).
(ii) Ammino-complexes (ligands are molecules of ammonia or organic ammines – [Ag(NH3)2]Cl).
(iii) Hydroxy-complexes (ligands are OH– anions – Na2[Sn(OH)4]).
(iv) Carbonyl-complexes (ligands are molecules of carbon monoxide – [Fe(CO)5]).
(v) Acido-complexes (ligands are anions of inorganic acids). Examples: chlorocomplexes K2[HgCl4], fluorocomplexes K3[FeF6], cyanocomplexes KFe[Fe(CN)6], thiocyanocomplexes K3[Fe(SCN)6], sulphitocomplexes K[Ag(SO3)], etc.](/documents_6/ec394fe2962c5c2a163839d96200d6bc/img15.jpg)
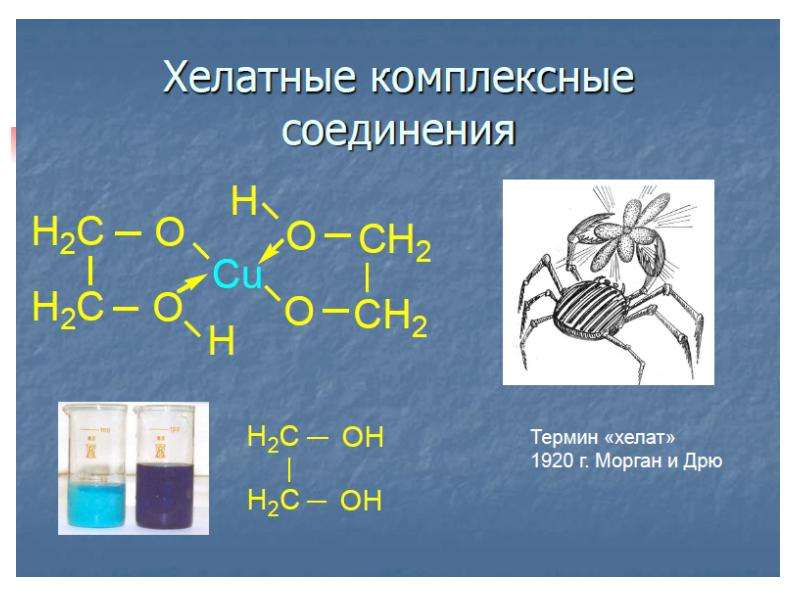
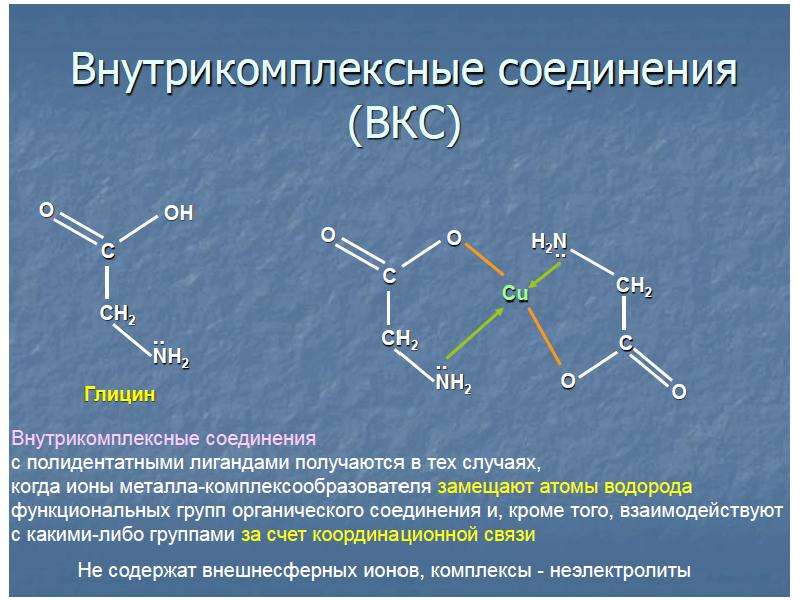
![б)Гидроксокомплексы – это комплексные анионы, в которых лигандами являются гидроксид-ионы OH–. Комплексообразователями являются металлы, склонные к проявлению амфотерных свойств – Be, Zn, Al, Cr.
б)Гидроксокомплексы – это комплексные анионы, в которых лигандами являются гидроксид-ионы OH–. Комплексообразователями являются металлы, склонные к проявлению амфотерных свойств – Be, Zn, Al, Cr.
Например: Na[Al(OH)4], Ba[Zn(OH)4].
в) Аммиакаты – это комплексные катионы, в которых лигандами являются молекулы NH3. Комплексообразователями являются d-элементы.
Например: [Cu(NH3)4]SO4, [Ag(NH3)2]Cl.](/documents_6/ec394fe2962c5c2a163839d96200d6bc/img16.jpg)



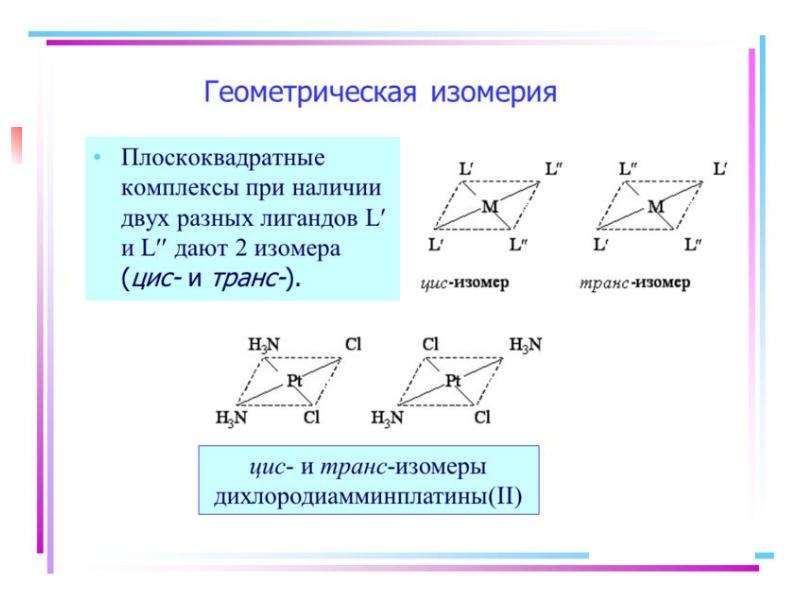
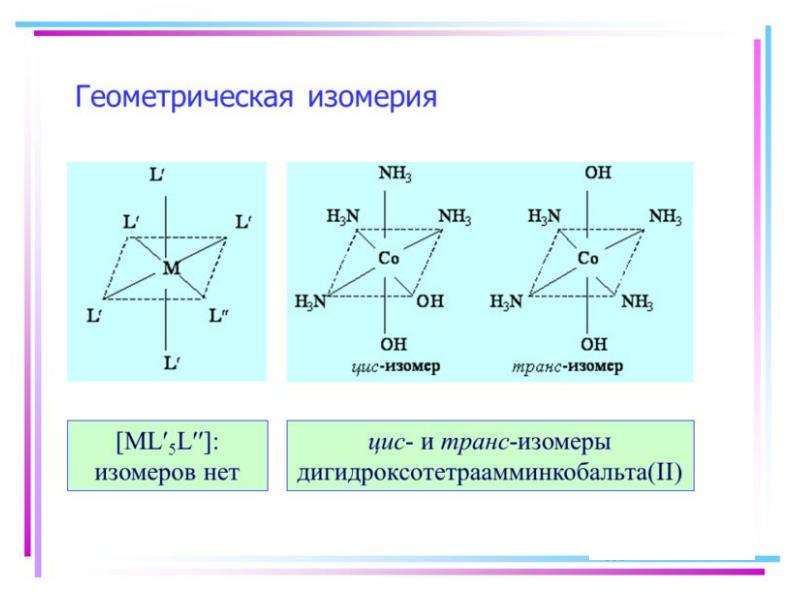
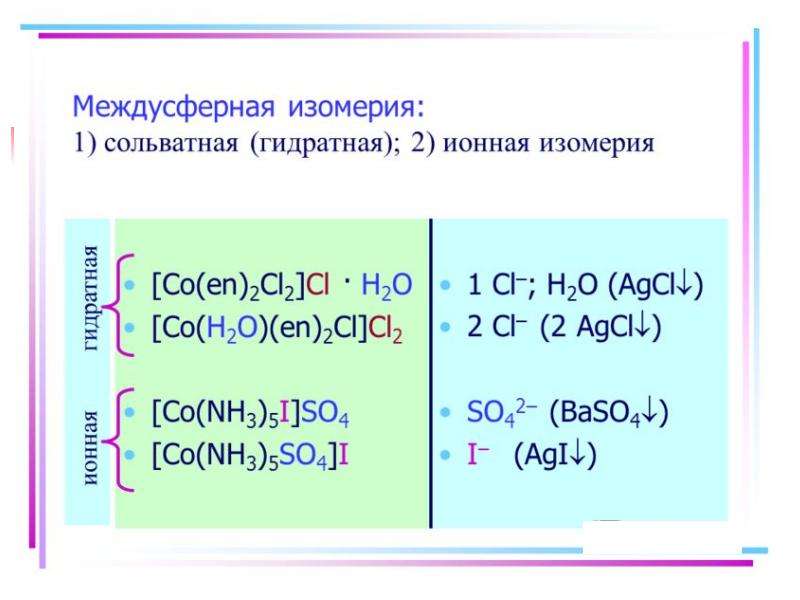


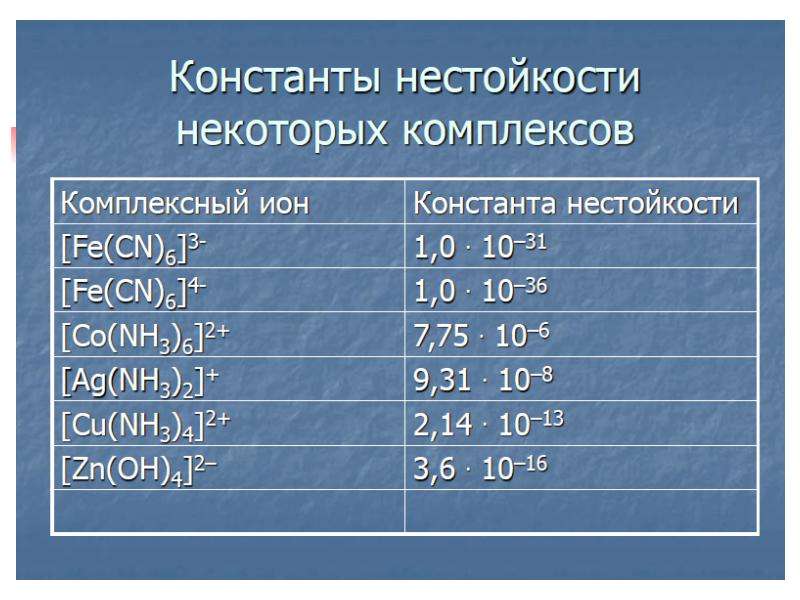
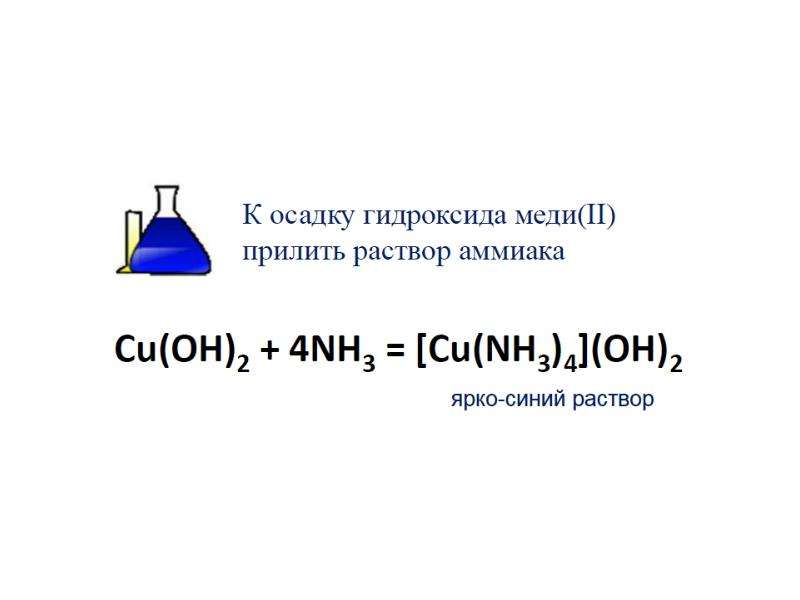
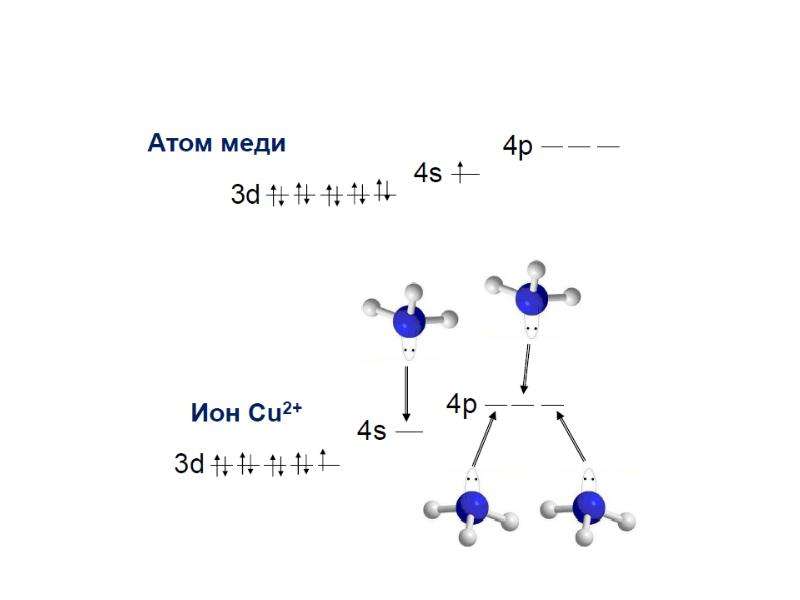
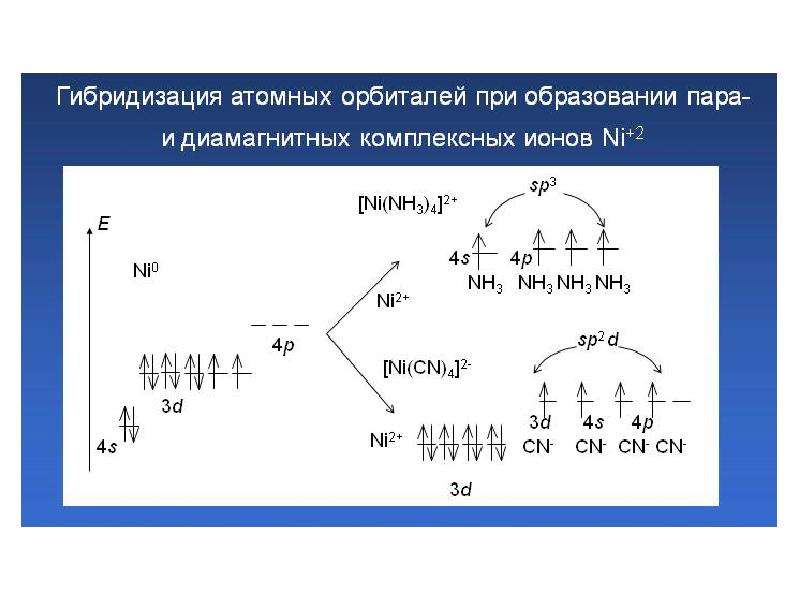
![Electronic structure of complex ions
Interaction of lone electronic pairs of ligands with empty valence orbitals of the central ion of different types leads to their hybridization. For example, the electronic structure of a complex ion [Cu(NH3)4]2+ can be reflected as following:](/documents_6/ec394fe2962c5c2a163839d96200d6bc/img29.jpg)

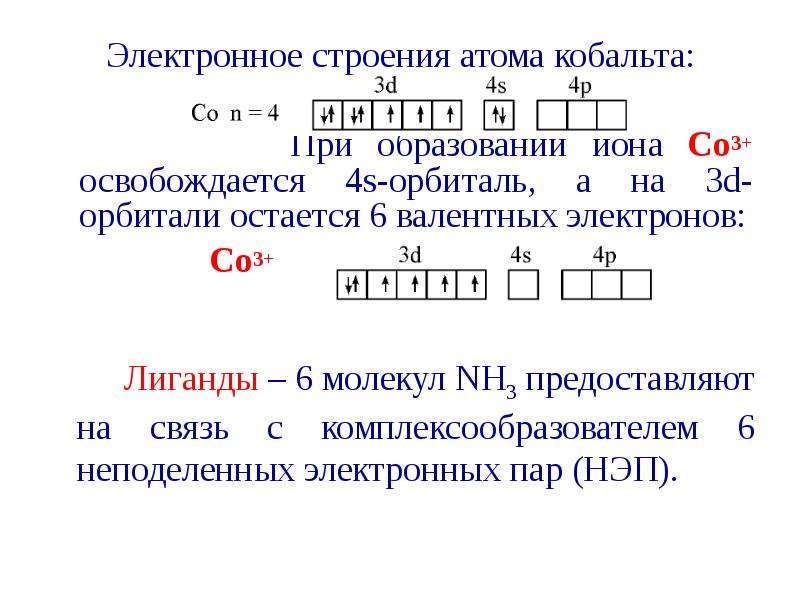

![Все валентные электроны спарены. Комплекс [Co(NH3)6]3+ - диамагнитный, что согласуется с экспериментом.](/documents_6/ec394fe2962c5c2a163839d96200d6bc/img36.jpg)